Photon Hammer™ Substrates (PHS) for Protein Crystallization
Photon Hammer™ Substrates (PHS) for Protein Crystallization
1. Introduction to the Photon Hammer Substrates (PHS)
Parallel Synthesis Technologies, Inc. is very pleased to offer its unique Photon Hammer heterogeneous nucleation technology to facilitate the crystallization of proteins in a convenient self-contained cartridge. Using a new patent-pending laser processing technique called the Photon Hammer, myriad micro-cracks are fabricated within a small, spatially well-defined region of a glass substrate which, when placed in contact with a protein solution to be crystallized, can act as potent heterogeneous nucleants for the crystallization of proteins. Photon Hammer Substrates (PHS) cartridges are now available for all familiar protein crystallization experiment protocols including Vapor Diffusion, Liquid Diffusion and GPCR in LCP.
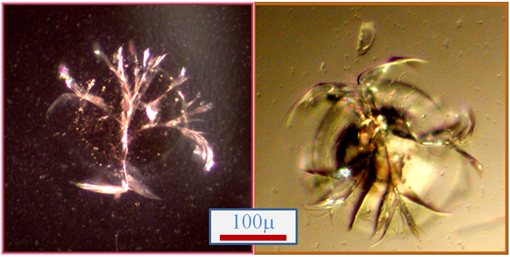
Fig. 1 Images of bare, unreacted heterogeneous nucleants prepared with using the Photon Hammer laser processing technology. The nucleation sites, each of which possesses countless microscopic cracks, are present within each well of the Photon Hammer Substrates (PHS) cartridge. Note that when the cracks emanating from the central damage zone propagate into the undamaged substrate the cracks are deflected and terminate thereby limiting the nucleant to a small, spatially well-defined area. Each variable width crack tapers from a finite width in the damage zone to zero width at its termini both perpendicular and parallel to the plane of the substrate. Therefore, cracks ranging in size from microns to zero width are present within each nucleant. We hypothesize that protein molecules may lose translational motion and begin ordering when absorbed into a crack of an appropriate size and shape thereby forming the first stages of an incipient crystal nucleus.
The Photon Hammer heterogeneous nucleants are fabricated from glass. A glassy material is particularly well suited to the formation of cracks. If cracks are initiated in a crystalline material it would likely propagate along the cleavage planes through the substrate resulting in mechanical failure of the material into pieces. Likewise, polymers may melt or decompose and metals are damaged in ways that do not result in fractures. As shown in Fig. 1, the cracks initiated from the laser pulse terminate a short distance from the damage zone. Unlike the “debris-based” heterogeneous nucleants, which are comprised of bits of broken or ground glass, horsehair, polymer spheres for seeding, seaweed and the like, the Photon Hammer technology provides a distinct, integral and well-localized heterogeneous nucleant. Since the nucleant is integral to PHS slide, it does not require any manipulation, loading into the crystallization trial or separation from your protein crystals after growth like the debris-based nucleants.
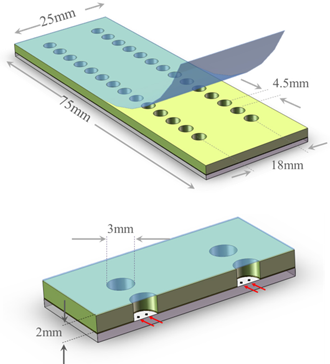
To provide contact between the nucleant and the protein solution polymer wells configured on a 384-well format are laminated onto these glass heterogeneous nucleation substrates to provide convenient 25mm x 75mm Photon Hammer Substrate (PHS) slide cartridges in which the protein crystallization experiments are performed (see below). You just select the type of crystallization protocols desired (vapor diffusion, liquid diffusion or GPCR/LCP), dispense your solutions and precipitants into the wells manually or automatically and seal. Crystallization progress is monitored from either side of the transparent Photon Hammer Substrate cartridge. With only minor changes to your current crystallization protocols you can take advantage of the Photon Hammer heterogeneous nucleation technology and, with free screening buffers shipped with each PHS purchase, it may be possible to reduce your screening costs.
2. Photon Hammer Substrates (PHS)
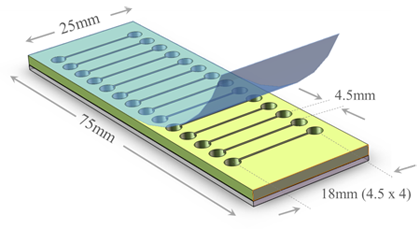
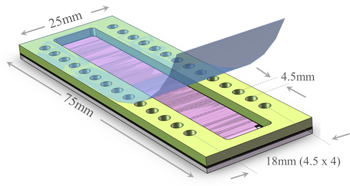
Fig. 2 A Photon Hammer Substrate (PHS-VD) cartridge, which is used for vapor diffusion crystallization trials, contains integral heterogeneous nucleants within each of 14 wells. The PHS-VD slide has the nucleants on a glass slide (grey) onto which the acrylic wells (yellow) have been laminated. The protein solutions are placed into one row and the vapor equilibrant solution placed into the wells of the adjacent row. The cartridge is tape sealed (blue) and the small channel connecting the two wells allows the water vapor to equilibrate between them.
To screen your protein crystallization conditions with the heterogeneous nucleants contained within the PHS you simply add your protein solution into a well and the precipitant or buffer solution into an adjacent well and seal the wells. The wells are interconnected by either a liquid channel (for Liquid Diffusion experiments) or a vapor channel (for Vapor Diffusion trials). Crystal growth is monitored from either side of the transparent cartridge and the crystals may be harvested by simply cutting the tape seal and removing the crystals. For the growth of GPCR materials, the membrane protein is mixed with LCP or other media (see below), dispensed into the well and the sample sealed.
The below sections describe the PHS, how the cartridges are used for protein crystal growth, the types of screening crystallization protocols that may be performed, how the cartridges are stored as well as the viewing and harvesting the protein crystals.
2.A Vapor Diffusion with Photon Hammered Substrates (PHS-VD Cartridges)
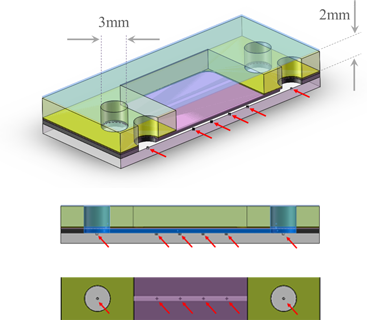
Fig. 4 A Photon Hammer Substrate (PHS-LD) cartridge, which is used for liquid-liquid diffusion crystallization trials, contains integral heterogeneous nucleants within each of 14 wells. The PHS-LD slide has the nucleants on sealed and solutions allowed to equilibrate a glass slide (gray) onto which the acrylic wells (yellow) have been laminated with PSA (black). The protein solutions are placed into one row and the precipitant placed into the wells of the adjacent row. The cartridge is tape sealed (blue) and the small channel connecting the two wells allows the two solutions to slowly intermix.
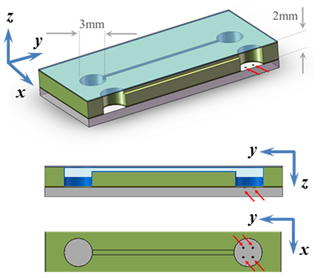
Fig. 3 In the PHS-VD slide, the protein solution in a well (volume = 10-15μL) containing the heterogeneous nucleants (red arrows) and the equilibrant in the adjacent well are connected by a 100μm x 100μm x 15mm vapor conducting channel (channel not shown to scale). After sealing with tape, the solutions equilibrate and the crystals harvested by removing or cutting the tape.
For pricing and ordering information please see the Purchase page on this website. Please remember that when you purchase either the PHS-VD or PHS-LD substrates you will receive free crystallization screening buffers – you’ll never need to purchase screening buffers again.
2.B Liquid Diffusion with Photon Hammered Substrates (PHS-LD)
The liquid diffusion taking place within the PHS-LD slides (Fig. 4), where the precipitant
Fig. 5 Sectional views of wells in adjacent rows in the PHS-LD slide showing the two wells, which contain the protein solution and precipitant, connected by a channel (200μm x 250μm x 15mm) which allows the solutions to slowly interdiffuse (channel not shown to scale). The heterogeneous nucleants (red arrows) in the glass layer are present in both wells and the channels. Crystal growth may be viewed form the top or bottom of the slide and crystals harvested by simply cutting the tape.
Please remember that whether you are purchasing the PHS-VD for vapor diffusion experiments or screening samples with the PHS-LD liquid diffusion cartridges that you receive free crystallization buffers with each PHS order.
2.C Crystallizing GPCR in Lipidic Phases with Photon Hammered Substrates (PHS-GPCR Cartridges)
Fig. 6 A Photon Hammer Substrate (PHS- GPCR) cartridge, which is used for crystallization screening trials for GPCR and other membrane proteins, contains integral heterogeneous nucleants within each of 28 wells of the PHS-GPCR. The GPCR is mixed with some lipidic material, dispensed into the well and onto the nucleant and the wells sealed with tape. Crystal growth is monitored from the top or bottom of the PHS slide and any suitable crystal isolated by simply cutting or removing the tape seal.
Using the PHS-GPCR Cartridges Once the oleophilic protein is dispersed into the lipidic phase chosen for the crystallization trial the protein-lipidic phase dispersion is dispensed into one of 28 wells on the PHS-GPCR slide and the slide tape sealed. Each of these wells, which possess integral heterogeneous Photon Hammer nucleants on the glass well bottom, has a total volume of approximately 14µL. The wells may be inspected for crystal growth from the top or bottom of the slide cartridge and the crystals removed from the well when ready to harvest by simply cutting the tape seal.
These same PHS-GPCR slides can be used to for GPCR diffusion screening (i.e., to determine the diffusion rate of the membrane protein in the lipidic phase). If the GPCR is not observed to diffuse within the lipidic phase according to some predetermined parameters, then there would be no need to set up screening trials for a protein that cannot diffuse within the crystallization matrix.
2.D Use of Automated Dispensing with the Photon Hammer Substrates
These PHS-GPCR cartridges can be easily configured for use with automated liquid or GPCR dispensing protocols as depicted in Fig. 7. As shown in Figs. 2, 4 and 6, the wells on any PHS slide are related by multiples of the 4.5mm spacing of the SBS 384-well format. To facilitate handling by automated dispensing equipment Parallel provides a simple inexpensive adapter to allow four 25mm x 75mm PHS slides to be robotically handled and as SBS-formatted microtiter plate.
2.E Custom Photon Hammer Substrates
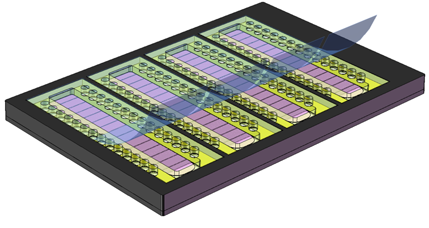
Fig. 7 Four PHS slides can be placed into an adapter that may be manipulated by automated liquid dispensers as an SBS-formatted microtiter plate.
3. Protocols and Methods
The section describes how to simply, quickly and conveniently load all types of PHS cartridges. The sample and precipitant/buffer are pipetted into adjacent wells, which are in communication via a water vapor-conducting conduit for the PHS-VD slides and a liquid bridge for the PHS-LD slides, and the slide sealed to await crystal growth as the two solutions equilibrate.
3.A Vapor Diffusion Experiments with PHS-VD Cartridges
Using the PHS loading fixture (please see Purchase), or a suitable holder from your laboratory, the PHS-VD slide is used to screen protein crystallization conditions as follows:
- One of two types of PHS-VD slide is selected depending on the volume of equilibrant desired: the PHS-VD-15 has a protein sample well of 15µL volume and an equilibrant well of 15µL or the PHS-VD-50 has a sample well of 15µL and an equilibrant well of 50µL volume;
- The PHS-VD slide is placed into the PHS loading fixture, the chosen buffer tubes into the buffer slots and the protein tubes into the chilled aluminum block.
- The row of wells along one side of the slide, which contain the PH nucleants, is filled with the protein solution;
- There are Photon Hammer heterogeneous nucleants in the well containing the protein solution only. Make sure the protein solution is placed into the well with the PHS nucleant present;
- The wells should not be filled more than ~70% full to avoid sealing issues;
- The row of wells along the other side of the slide is filled with the equilibrant solutions;
- The backing is removed from the precut sealing tape and the tape used to seal the wells;
- Crystal growth may be monitored optically from either surface of the slide;
- To harvest the developed crystals the tape seal is simply cut or removed for retrieval
3.B Liquid Diffusion Experiments with PHS-LD Cartridges
Using the PHS loading fixture (please see Purchase), or a suitable holder from your laboratory, the PHS-LD slide is used to screen protein crystallization conditions as follows:
- The PHS-LD slide is placed into the PHS loading fixture, the chosen buffer tubes into the buffer slots and the protein tubes into the chilled aluminum block.
- The row of wells along one side of the slide is filled with the protein solution;
- Since both rows of wells and the intervening liquid channel all possess PH nucleants it makes no difference which row of the PHS-LD slide is selected for the protein and which row for the precipitant.
- The wells should not be filled more than ~70% full to avoid sealing issues;
- The row of wells along the other side of the slide is filled with the equilibrant solutions;
- The backing is removed from the precut sealing tape and the tape used to seal the wells;
- Crystal growth may be monitored optically from either surface of the slide;
- To harvest the developed crystals the tape seal is simply cut or removed for retrieval
3.C GPCR Diffusion Experiments with PHS-GPCR Cartridges
Using the PHS loading fixture (please see Purchase), or a suitable holder from your laboratory, the PHS- GPCR slide is used to screen protein crystallization conditions as follows:
- If desired, the GPCR or membrane protein can first be screened to see if the protein has a sufficiently high diffusion rate within the lipidic phase to allow subsequent crystal growth to occur at a reasonable rate. This is accomplished by placing a very small concentrated GPCR sample in the well and covering the protein with dispensed lipidic phase. If the protein does not diffuse into the surrounding lipidic phase at some predetermined rate, then this sample will always be incapable of crystallizing and can be eliminated from further screening trials.
- A suitable GPCR or membrane protein is mixed a Lipidic Cubic Phase (LCP) or some other lipidic phase.
- The PHS- GPCR slide is placed into the PHS loading fixture.
- All wells contain heterogeneous nucleants and all wells are filled with a mixture of the GPCR and lipidic phase.
- The wells should not be filled more than 80% full to avoid sealing issues;
- The backing is removed from the precut sealing tape and the tape used to seal the wells;
- Crystal growth may be monitored optically from either surface of the slide;
- To harvest the developed crystals the tape seal is simply cut or removed for retrieval
4. Protein Crystal Image Gallery
In the Image Gallery section, images of protein crystals are shown whose growth has been influenced in various ways by the Photon Hammer heterogeneous nucleants. The sections are arranged according to what type of heterogeneous nucleation effect was observed (see list of effects below). When browsing the image gallery clicking an image load a larger view and mousing over the larger image zooms in and allows panning about the image.
A heterogeneous nucleant (HN) can influence crystal growth in many ways but the fundamental effect must manifest itself in creating one and only one type of nucleus at the earliest stages of nucleation and crystal growth. There are several examples given below where it is clear that once a given type of nucleus forms other crystalline polymorphs or nuclei of differing habit cannot form and are completely suppressed. In all of the examples shown in the Image Gallery, which provides a summary of hundreds of heterogeneous nucleation experiments, there was never an example of two different crystal habits, types or polymorphs observed growing within the same solution.
A heterogeneous nucleant is a solid state material which, when in contact with a solution containing the soluble species to be crystallized, alters the course of crystallization as compared to the course in the absence of the HN in ways such as:
- A higher percentage of crystals form in screening trials with HN than without HN
- Crystals form faster in the presence of a HN than without HN
- Crystals nucleate and form on the region of the substrate where the HN is physically located and not on other regions of the same substrate
- Crystal of a different habit or polymorph form in the presence of a HN but not in the absence HN
- Crystallization, or a particular crystalline polymorph, is suppressed in the presence of a heterogeneous nucleant
- Fewer larger crystals, rather than more smaller crystals, form in the presence of a HN but not in the absence HN
- Higher quality crystals form in the presence of a HN than without HN
- Crystals that have proven difficult or impossible to crystallize crystalize more readily in the presence of an HN
5. Crystallization of Non-proteinacious Materials
While there is a very great importance attached to the crystallization and structure determination of proteins, which is obvious because of their scientific, technical and commercial attributes, crystals of non-proteinacious materials have received far less attention. We have found that the enhanced nucleation effects associated with the Photon Hammer Substrates not only increases the efficacy of the protein crystallization process but likewise also exhibits a strong nucleating effect on a variety of other materials undergoing crystallization from solution. Although there are many technology areas, such as polymorph control of crystals of small molecule drugs or purification by crystallization, where influence on the speed, selectivity and reproducibility of a crystallization process could be of great benefit, the influence of heterogeneous nucleants on the growth of these materials is far less studied.
Since the cracks that are responsible for increased efficacy of the Photon Hammer heterogeneous nucleants taper to zero width as the terminate with the glassy substrates with the PHS cartridges, no matter how small the incipient unit cell may be there is a crack of similar dimensions since all molecular dimensions are present with the Photon Hammer nucleants.
Please see the Image Gallery for some beautiful and fascinating images of inorganic materials and small molecules nucleated on Photon Hammer Substrates.
6. Ordering the PHS and Accessories
Please visit the Purchase section of this website for the PHS slides, accessories and your free crystallization screening buffers.